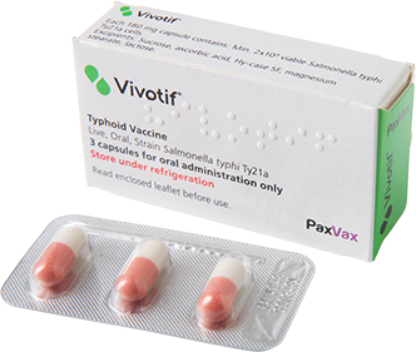
What Is VIVOTIF1?
VIVOTIF is indicated for active oral immunisation against typhoid fever, caused by Salmonella enterica serovar Typhi (S. Typhi), in adults and children aged five years and older.
This vaccine should be used in accordance with official recommendations.
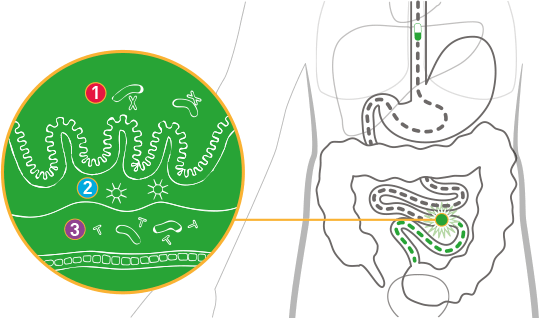
VIVOTIF works in three ways
VIVOTIF protects against Salmonella Typhi by mimicking the response to natural infection and stimulating local, cellular and systemic/humoral immunity 2-5
- Local immune response: IgA
- Cellular mediated immunity:
macrophages/cytotoxic T lymphocytes - Systemic/humoral immune responses: IgG